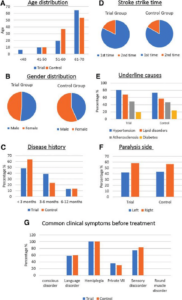
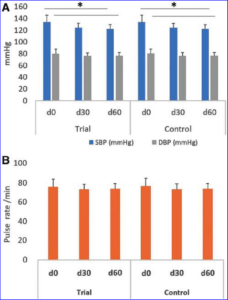
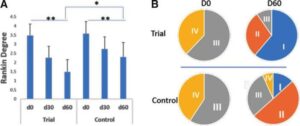
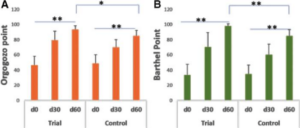
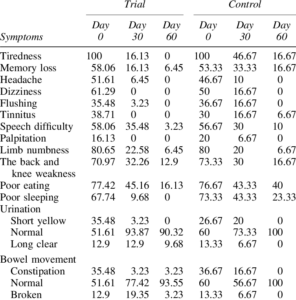
Stroke remains a major cause of human disability worldwide. Interventions and rehabilitation at the poststroke stage are critical for recovery. A single-blinded randomized controlled trial was conducted on 61 patients diagnosed with subacute stage of ischemic stroke. Ingestion of Nattospecs was tested as an adjuvant to support rehabilitation when combined with standard of care (SOC) treatment (electroacupuncture and Naatrapyl) (Trial group) and compared to SOC treatment alone (Control group). After 60 days, results showed that both Trial and Control groups achieved significant improvements in physical activities, blood pressure control, serum lipid panels, and quality of life. Nattospes as a food supplement has good supportive effects on treatment and rehabilitation after ischemic stroke by showing statistically significant improvement of stroke-related symptom in scores from modified Rankin, Orgogozo, and Barthel scales. In addition, Nattospes showed a good safety profile, with no adverse effects reported in both clinical and paraclinical parameters. This study indicated that Nattospes as nutraceutical supplement can be applied safely and effectively in the management of subacute stage ischemic stroke. The findings of the study may also encourage further extensive clinical trials to fully explore the prospect of Nattospes as a nutraceutical adjunct in the management of cardiovascular disease.

 2021 Inventive Medical Foundation, All rights reserved
2021 Inventive Medical Foundation, All rights reserved